WHYCHOOSE US
PRODUCTHIGHLIGHTS
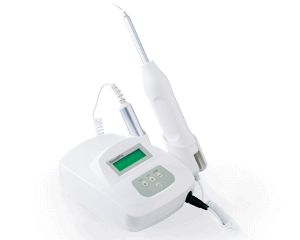
Ozone DTA
Bringing on the dentistry revolution with innovative ozone therapyO3 decomposition design
cost down, safety up
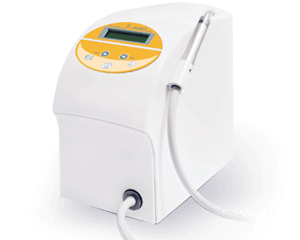
Ozone DTA J-500 Plus
Ozone Therapy + Disinfector = 2 in 1 DeviceO3 decomposition design
cost down, safety up

Ozone V-600 Mini
Vacuum Ozone deviceDiscover your Favorite Disinfector!
No need of Consumables!
Without Heat & Water!
O3 decomposition design
cost down, safety up

AD7 Mini Autoclave
A MUST-HAVE portable sterilizer specially designed for the handpiece!
INNOVATION
APOZA strives to discover more holistic, innovative and comprehensive solutions to enhance dental and medical operating experiences.
LATESTNEWS
2023.04.11
Registered in EUDAMED database
As below products have been registered in EUDAMED database. Click link for further details:
2022.12.20
CE certified products
OzoneDTA、OzoneDTA J-500、OzoneDTA J-500 Plus

2022.12.15
Brand-new launch: AD7 Mini Pro
Mini rapid steam sterilization autoclave